Investment Highlights
Bod Science (ASX:BOD) is a cannabis focused drug development and product innovation company.
ASX:BOD


The acquisition of Aqua Phase has been a strategic and methodological process, with a number of key milestones along the way.
We are increasingly confident in the commercial potential of this unique delivery format in numerous global markets.
Aqua Phase promises to enable poor solubility medications to become cheaper and safer as a result of lower side effects and less use of the active ingredient required due to the medications increased solubility. The solubility data we have already demonstrated from the UV analysis enables us to move a number of commercial opportunities in cannabis and non-cannabis medications forward with increasing confidence.
Jo Patterson CEO
Competitive Landscape
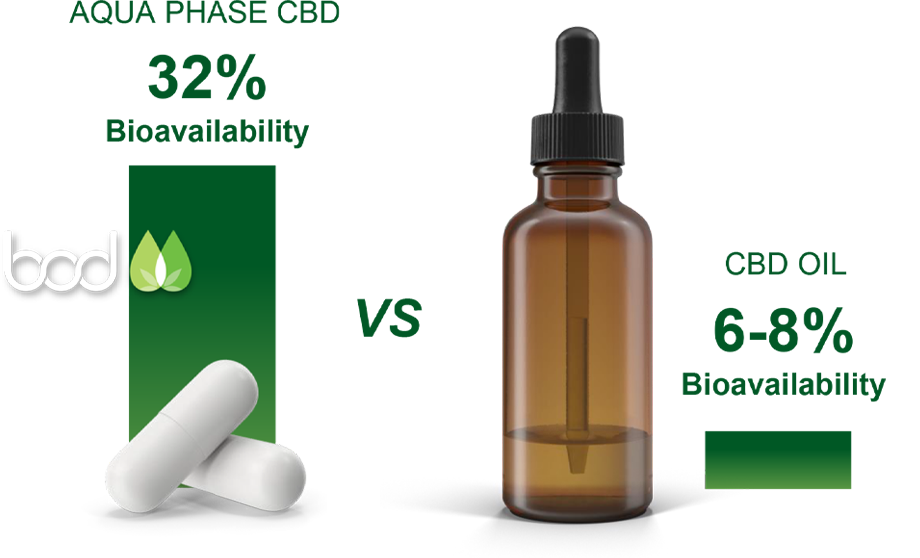
Ultraviolet (UV) Analysis of Solubility
Aqua Phase CBD solubility ranges 1.6 millimolar (mM) to 2.7mM under various conditions
VS
Standard CBD solubility is 0.2 micromolar (μM)
| Aqua Phase | Emulsifiers | Nano Technology | Phospholipids | |
| Water Soluble vs Dispersible | Water Soluble | Water Dispersible | Water Dispersible | Water Dispersible |
| Delivery Platform | Modified Search | Phospholipid | Phospholipid | Phospholipid |
| Format | Powder | Liquid | Liquid | Liquid |
| Bioavailability |

Aqua Phase CBD Combination
CBD
The exact proportions of active (CBD) and substrate (starch based molecule) are defined
Heat and agitation
Starch Based Molecule
Specific temperatures and the agitation process are set Invention offers an API that is flavourless, colourless and stable
Bioavailable
CBD
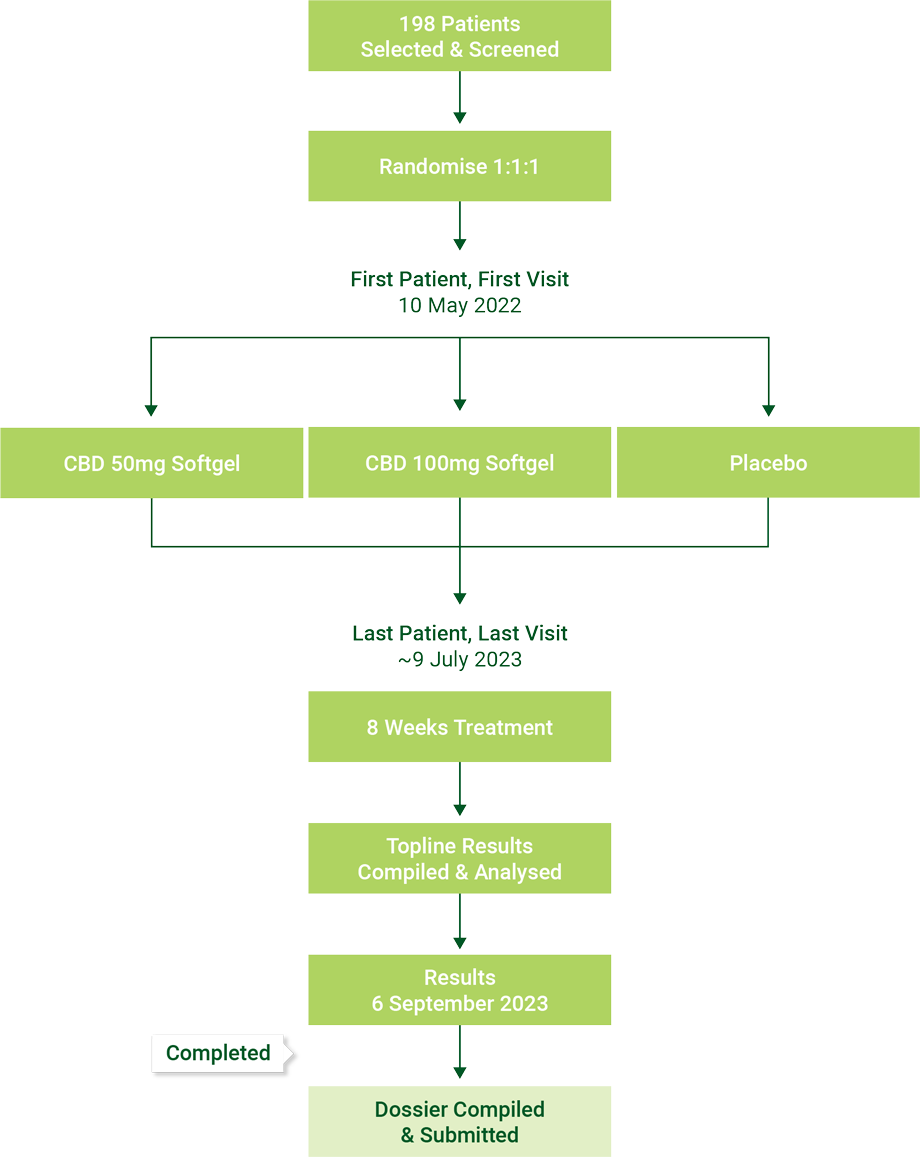
Phase IIB Insomnia Clinical Trial for Schedule 3 CBD Medication
Bod anticipates to deliver the first over-the-counter (OTC) Cannabis product to be submitted to the TGA for an ARTG registration.
Successful receipt of ARTG registration will elevate Bod to be one of only two companies globally to have a registration of a Cannabis product (Jazz Pharma).
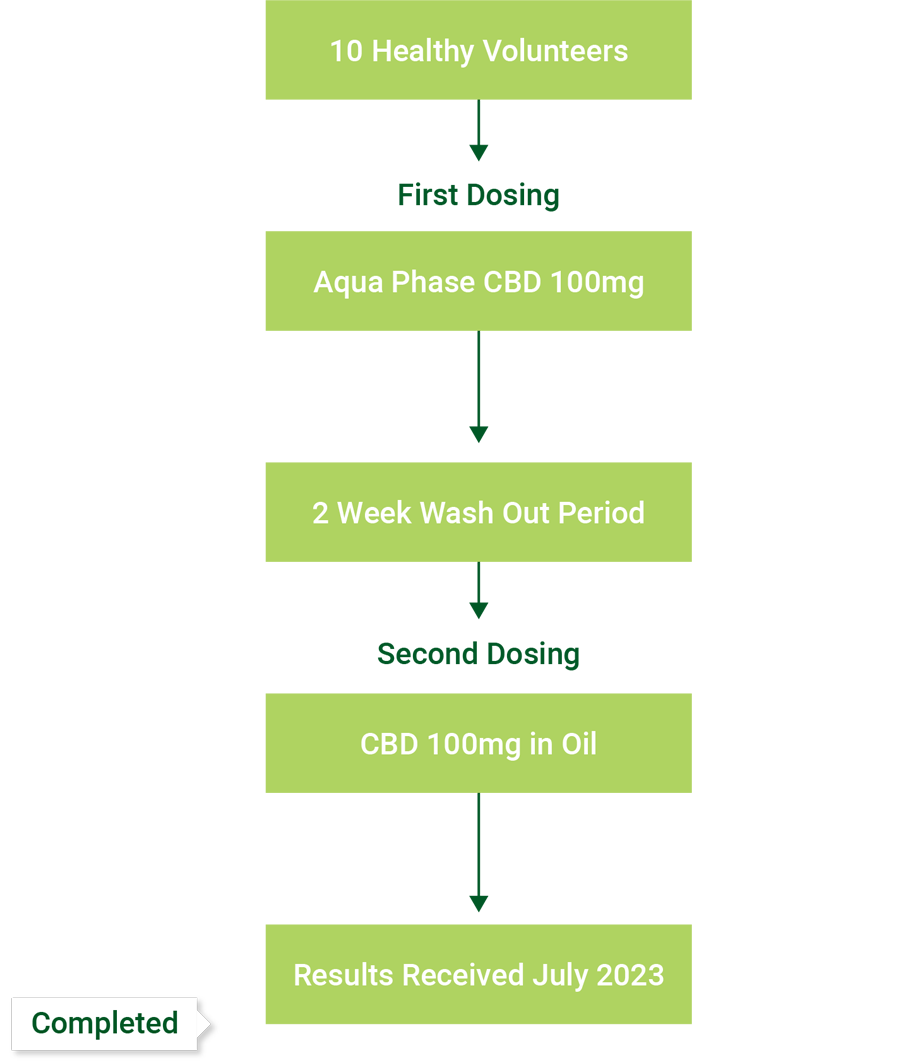
- In conjunction with The Woolcock Institute, Bod is undertaking a clinical trial testing the efficacy of Bod’s CBD ECS Bioabsorb Softgel (utilising Bod’s Aqua Phase Technology) vs Epidiolex CBD Oil Solution when used to treat insomnia, to follow with submission for a Schedule 3 CBD medication
- ARTG registration is granted under the TGA regime. It requires the submission of a dossier, including results of a gold standard clinical trial (double blinded placebo) and efficacy results relating to the primary end point
- An approval will have a significantly beneficial impact on access for customers / patients with insomnia. The product will no longer require a prescription and will be available through pharmacy (OTC)
- It will set the benchmark for future studies and submissions to the TGA and registrations of Cannabis products
- It is anticipated achieving a successful trial, along with being the first registered cannabis CBD product in Australia will have a significant and positive overall impact on the Australian Cannabis industry

Phase IIB Insomnia Clinical Trial
for Schedule 3 CBD Medication